Design of Pharmaceutical Manufacturing EquipmentBusiness Introduction

Design of Pharmaceutical Manufacturing Equipment
Pharmaceutical manufacturing plants require numerous processes, ranging from the production of the medicine itself to its packaging, and must incorporate various functions.
Tokyo Densei Industrial leverages its accumulated technology and expertise to provide not only the functions that customers want as standalone machines or systems, but also to enhance them with added value, ensuring superior operability and maintainability.
Furthermore, we adhere to strict guidelines for verification and documentation (validation) and offer comprehensive support throughout the entire process—from engineering and design to manufacturing, construction, supervision, and maintenance.
Service Offerings
- Proposals and procurement of production equipment and ancillary equipment
- System engineering, including:
- DCS/MES/SCADA systems/Monitoring systems/PLC・HMI systems
- Electrical and instrumentation work (design, construction, and supervision)
- Delivery and installation work of production equipment and machinery
- Validation services (DQ/IQ/OQ)
- Calibration of instrumentation devices and weighing machines, etc.
- Various maintenance services
Features
- Compliance with CSV (Computerized System Validation)
We establish and implement rules for the development, verification, operation, and decommissioning of computerized system validation, ensuring all activities are conducted accordingly and results are documented.
- Compliance with 21 CFR Part 11 (Title 21 Code of Federal Regulations, Part 11)
We support electronic records and electronic signatures in accordance with regulatory requirements.
- Compliance with DI (Data Integrity)
Our DI-compliant manufacturing data management system provides a comprehensive solution for key DI compliance challenges, including ID and password management, time synchronization, audit trails, and data management.
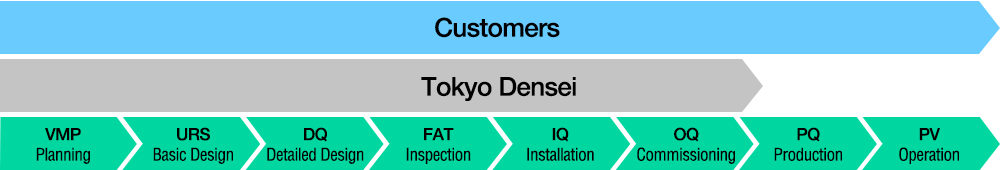
The Process from Design to Operation
We provide comprehensive support throughout the entire process—from design and production to construction, supervision, and maintenance—ensuring verification and documentation (validation) in compliance with strict guidelines.
Common Inquiries
We frequently receive inquiries such as the following.
If you have any issues or concerns regarding your production or ancillary equipment, please feel free to contact us.
- We want to upgrade a pharmaceutical manufacturing plant that has been in operation for XX years to comply with PIC/S GMP standards.
- We want to renovate an old plant and convert it into a completely different pharmaceutical manufacturing line.
- We aim to achieve a fully paperless operation. However, while the system handles monitoring and report generation, we still need to print documents for signatures. Additionally, certain elements, such as attaching trend graphs to paperless recorders, are not yet centrally managed.
- In SCADA and other systems, if the system (*) is not running, alarms are not triggered, preventing real-time notifications from reaching the appropriate personnel.
Example: Logging into a client PC and keeping the application running at all times
- We want to minimize the risk of system failures and data loss.
→Redundancy is possible.
(If one system fails, the backup system will automatically take over, ensuring continuous production.)
Track Record of Implementation
- Company A: Master PLC system / weighing system / production equipment installation / instrumentation work
- Company B: Master PLC system
- Company C: Master PLC system / predictive maintenance data system / instrumentation work
- Company D: Manufacturing data management system
- Company E: Master PLC system
- Company F: Preparation system / instrumentation work
- Company G: IoT G/W system (confirming G/W)
- Company H: Secondary-side instrumentation work for production equipment